What
are zeolites?
|
General
Zeolites
are among the most common products of chemical interaction between
groundwaters and the Earth’s crust during diagenesis and
low-grade metamorphism. The unique crystal structures of zeolites
result in large molar volume, high cation-exchange capacities, and
reversible dehydration (Neuhoff et al. 2000).
The zeolite mineralogy of low-grade meta-basalts is a sensitive indicator for the thermobarometric evolution of oceanic crust and continental flood basalts. Discrete temperature-, pressure-, and depth controlled zones characterised by assemblages with silica minerals, phyllosilicates and zeolites frequently serve as metamorphic indicator in low-grade meta-basalts. Natural zeolites commonly form at low temperatures by reactions of aqueous solutions with volcanic rocks, and volcanogenic sediments. Consequently, ion-exchange capabilities of zeolites can affect ground water composition and quality in volcanic aquifers (e.g. White et al 1980). History
and definition of zeolites
The
Swedish mineralogist CRONSTEDT introduced the term
“zeolite” in 1756 for certain silicate minerals in
allusion to their behaviour on heating in a borax bead (Greek: zeo = to
boil; lithos = stone). Hey (1930) concluded that zeolites in general
have aluminosilicate frameworks with loosely bonded alkali or
alkali-earth cations, or both. He pointed out the consequential
requirements that the molar ratio Al2O3
: (Ca, Sr, Ba, Na2,
K2)O = 1 and that O : (Si + Al) = 2 in the
empirical formula.
Another characteristic features of zeolites are the potential for reversible low-temperature dehydration, the ability of dehydration and reversibly absorption of other molecules. Definition of zeolites after the International Mineralogical Association, Commission on New Minerals and Mineral Names (Coombs et al. 1998): “A zeolite mineral is
a crystalline substance with a structure characterized by a framework
of linked tetrahedra, each consisting of four O atoms surrounding a
cation. This framework contains open channels and cages. These are
usually occupied by H2O molecules and extra-framework
cations that are commonly exchangeable. The channels are large enough
to allow the passage of guest species. In the hydrated phases,
dehydration occurs at temperatures mostly below about 400°C and
is largely reversible. The framework may be interrupted by (OH, F)
groups; these occupy a tetrahedron apex that is not shared with
adjacent tetrahedral” (Coombs et al. 1998).
Structure
The
basic feature of all zeolite structures is an aluminosilicate framework
(tectosilicate) composed of (Si, Al)O4
tetrahedra, each oxygen of which is shared between two tetrahedron
(ARMBRUSTER & GUNTER 2001). The net negative charge on the
tectosilicate framework is balanced by the incorporation of cations
(interchannel cations) in ~2 to 10 Å cages or channels. In
most cases Ca2+, Na+
or K+
and less frequently Li+,
Mg2+,
Sr2+
and Ba2+
are situated in cavities within the framework structures. This feature
can also be observed in feldspar and feldspathoid minerals. But in
contrast to this feldspar and feldspathoid minerals the zeolite
aluminosilicate framework contain open cavities and open
channels (i.e. they have lower densities) through which ions
can be either extracted or introduced ions (Armbruster &
Gunter 2001).
Their compositions are represented by the structural formula (1):
(A+z)y/z(B+3)y(Si)xO2(x+y)
·
nH2O
(1)
Where A
represent interchannel cations (such as Na+,
K+,
Ca2+,
Ba2+,
Sr2+,
Mg2+
and Fe2+),
B are tetrahedral coordinated trivalent cations in the zeolite
framework (Al3+
and Fe3+),
z is the charge on the interchannel cations, n is the number of moles
of interchannel molecular water, and x and y are the stoichiometric
coefficients for trivalent cations and Si4+ in tetrahedral sites,
respectively. The quantities y/z and 2(x+y) represent the
stoichiometries of the interchannel cations and framework oxygens,
respectively, necessary for maintaining charge balance in the
tectosilicate lattices of zeolites (Armbruster
& Gunter 2001).
An additional feature, which differentiated the zeolites still further from the feldspar and feldspathoid minerals, is the presence of water molecules within the structural channels. These are relatively loosely bound to the framework and cations, and like the cations they can be removed and replaced without disrupting framework bonds (Deer et al. 1992). Currently,
three classification schemes are used widely for zeolite structures.
Two of these are based upon specifically defined aspects of crystal
structure, whereas the third has a more historical basis, placing
zeolites with similar properties (e.g. morphology) into the same group (Armbruster
& Gunter 2001).
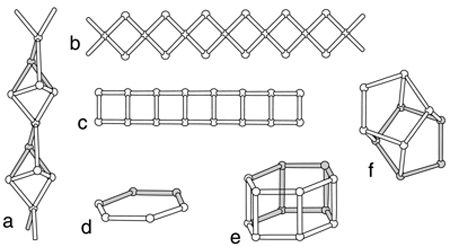
The first structural classification of zeolites is based on the framework topology, with distinct framework receiving a three-letter code (Meier et al. 1996). The second structural method for the classification of zeolites is based on a concept termed “secondary building units” (SBU). The primary building unit for zeolites is the tetrahedron and the SBUs are the geometric arrangements of tetrahedra (Breck 1974, Armbruster & Gunter 2001). Quite often, these SBUs tend to control the morphology of the zeolites. The third broad classification scheme is similar to the SBU classification of Breck (1974), except that it includes some historical context of how the zeolites were discovered and named. This scheme uses a combination of zeolite group names which have specific SBUs and is the widely used by geologists. This is the classification scheme used by Gottardi & Galli (1985) . Fig. 1 The structural
units, finite or infinite, which may be used to
assemble the frameworks of zeolites. a: The chain of fibrous zeolites;
b: The singly connected 4-ring chain; c: The doubly connected 4-ring
chain; d: The 6-ring (single); e: The 6-ring (double); f: The 4-4-1-1
heulandite unit. In each drawing, the balls represent tetrahedra
(SiO44- or AlO45-) and the bars represent oxygens
shared by the
tetrahedra (after Breck 1974 and Gottardi & Galli 1985).
Three
types of solid solution in zeolites are consistent with the
stoichiometry of formula (1). These solutions are not strictly coupled
and can occur independently from other substitutions as long as charge
balance is maintained.
The first of these is the solid solution within the tetrahedral sites. Tetrahedral substitution of Si4+ and Al3+ observed in zeolites is highly variable, whereas the substitution Fe3+ for Si4+ or Al3+ is limited (Neuhoff et al. 2000). Secondly, solid solutions among interchannel cations are often quite extensive, as evidenced by the large ion-exchange capacities of some zeolites (e.g. Colella 1996). Total interchannel ion charge is necessarily a function of Al3+ and Fe3+ content because the interchannel ions compensate for charge imbalances resulting from the presence of trivalent ions in tetrahedral sites. Zeolites with high Si/Al ratios commonly are richer in monovalent interchannel cations than are more aluminous samples of the same species. Twice as many monovalent ions as divalent ions are necessary to compensate for charge imbalances caused by Al3+ in the framework, and the additional monovalent ions often occlude H2O molecules present in isostructural zeolites with divalent interchannel cations (e.g. natrolite and scolecite, Ca-heulandite and Na-heulandite) (Neuhoff et al. 2000). The third type of solid solution in zeolites is a consequence of the loose bounding nature of molecular water in zeolites, whereas the total water content is a sensitive function of temperature, total pressure and the partial hydrostatic pressure (Neuhoff et al. 2000). Application
Zeolites
have many useful purposes. They can perform ion exchange, filtering,
odor removal, chemical sieve and gas absorption tasks. The most well
known use for zeolites is in water softeners. Calcium in water can
cause it to be "hard" and capable of forming scum and other
problems. Zeolites charged with the much less damaging sodium
ions can allow the hard water to pass through its structure and
exchange the calcium for the sodium ions. This process is
reversable. In a similar way zeolites can absorb ions and
molecules and thus act as a filter for odor control, toxin removal and
as a chemical sieve. Zeolites can have the water in their
structures driven off by heat with the basic structure left
intact. Then other solutions can be pushed through the
structure. The zeolites can then act as a delivery system for the new
fluid. This process has applications in medicine, livestock feeds and
other types of research. Zeolites added to livestock feed have been
shown to absorb toxins that are damaging and even fatal to the growth
of the animals, while the basic structure of the zeolite is
biologically neutral. Aquarium hobbyists are seeing more zeolite
products in pet stores as zeolites make excellent removers of ammonia
and other toxins. Most municipal water supplies are processed through
zeolites before public consumption. These uses of zeolites are
extremely important for industry, although synthetic zeolites are now
doing the bulk of the work.
|